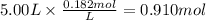The reaction 2N2O5 (g) → 2N2O4 (g) + O2 (g) has a reaction rate that is dependent only on the concentration of N2O5 and at a certain temperature has a rate constant k of 0.0168 s-1. If 2.50 moles of N2O5 were placed in a 5.00 liter container at that temperature, how many moles of N2O5 would remain after 1.00 min?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
The reaction 2N2O5 (g) → 2N2O4 (g) + O2 (g) has a reaction rate that is dependent only on the concen...
Questions

Chemistry, 11.03.2021 23:20

Engineering, 11.03.2021 23:20

Chemistry, 11.03.2021 23:20


Mathematics, 11.03.2021 23:20


Mathematics, 11.03.2021 23:20





Mathematics, 11.03.2021 23:20

Mathematics, 11.03.2021 23:20

English, 11.03.2021 23:20

Mathematics, 11.03.2021 23:20


Mathematics, 11.03.2021 23:20



Social Studies, 11.03.2021 23:20

![[N_2O_5] = [N_2O_5]_0 \times e^{-k \times t}](/tpl/images/0587/8861/ec7f1.png)
![[N_2O_5]_0](/tpl/images/0587/8861/4a20d.png) : initial concentrationk: rate constantt: time
: initial concentrationk: rate constantt: time![[N_2O_5] = 0.500 M \times e^{-0.0168 s^{-1} \times 60s} = 0.182 M](/tpl/images/0587/8861/ce720.png)