
Chemistry, 07.04.2020 23:26 gevaughn600
If the caffeine concentration in a particular brand of soda is 2.43 mg/oz, drinking how many cans of soda would be lethal? Assume that 10.0 g of caffeine is a lethal dose, and there are 12 oz in a can.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
If the caffeine concentration in a particular brand of soda is 2.43 mg/oz, drinking how many cans of...
Questions


Mathematics, 06.05.2021 18:10


Mathematics, 06.05.2021 18:10



Mathematics, 06.05.2021 18:10

Mathematics, 06.05.2021 18:10

Mathematics, 06.05.2021 18:10


Mathematics, 06.05.2021 18:10

History, 06.05.2021 18:10



Computers and Technology, 06.05.2021 18:10

Mathematics, 06.05.2021 18:10

Mathematics, 06.05.2021 18:10


Mathematics, 06.05.2021 18:10

Mathematics, 06.05.2021 18:10

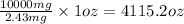
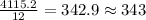 cans
cans

