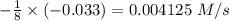Chemistry, 03.12.2019 16:31 madison1284
Consider the reaction: 8h2s(g)+4o2(g)→8h2o(g)+s8(g). δ[h2s]/δt = -0.033m/s. find δ[o2]/δt. δ[h2o]/δt. δ[s8]/δt. find the rate of the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Consider the reaction: 8h2s(g)+4o2(g)→8h2o(g)+s8(g). δ[h2s]/δt = -0.033m/s. find δ[o2]/δt. δ[h2o]/δ...
Questions



Social Studies, 23.04.2021 05:10


Mathematics, 23.04.2021 05:10


Mathematics, 23.04.2021 05:10

Mathematics, 23.04.2021 05:10






Mathematics, 23.04.2021 05:20



Computers and Technology, 23.04.2021 05:20


Social Studies, 23.04.2021 05:20

Mathematics, 23.04.2021 05:20

![\frac{[\Delta O_2]}{\Delta t} = -0.0165\ M](/tpl/images/0401/1414/d412a.png)
![\frac{[\Delta H_2O]}{\Delta t}= 0.033\ M/s](/tpl/images/0401/1414/ccd80.png)
![\frac{[\Delta S_8]}{\Delta t} = 0.004125\ M/s](/tpl/images/0401/1414/d55d1.png)
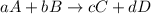
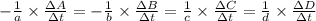
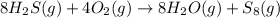
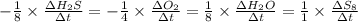
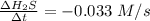
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t} =-\frac{1}{4}\times \frac{[\Delta O_2]}{\Delta t}](/tpl/images/0401/1414/fd6a9.png)
![-\frac{1}{8}\times (-0.33) =-\frac{1}{4}\times \frac{[\Delta O_2]}{\Delta t}](/tpl/images/0401/1414/02deb.png)
![-\frac{[\Delta O_2]}{\Delta t} = \frac{4}{8} \times (0.033) = 0.0165\ M](/tpl/images/0401/1414/323db.png)
![\frac{[\Delta O_2]}{\Delta t} = -0.0165\ M/s](/tpl/images/0401/1414/d516e.png)
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t} =\frac{1}{8}\times \frac{[\Delta H_2O]}{\Delta t}](/tpl/images/0401/1414/d13b5.png)
![\frac{[\Delta H_2O]}{\Delta t}=-\frac{8}{8}\times \frac{[\Delta H_2S]}{\Delta t}](/tpl/images/0401/1414/4e162.png)
![\frac{[\Delta H_2O]}{\Delta t}=-\frac{8}{8}\times (-0.033)](/tpl/images/0401/1414/b29cf.png)
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t} =\frac{1}{1}\times \frac{[\Delta S_8]}{\Delta t}](/tpl/images/0401/1414/1cad1.png)
![\frac{[\Delta S_8]}{\Delta t}=-\frac{1}{8}\times (-0.033)=0.004125\ M/s](/tpl/images/0401/1414/4b7e9.png)
![-\frac{1}{8}\times \frac{[\Delta H_2S]}{\Delta t}](/tpl/images/0401/1414/78436.png)