
Chemistry, 07.04.2020 23:36 rebeccamckellpidge
The combustion of propane gas is used to fuel barbeque grills. If 4.65 moles of propane, C3H8, are burned in a grilling session, how many moles of carbon dioxide gas are formed? C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(l)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1


Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1
You know the right answer?
The combustion of propane gas is used to fuel barbeque grills. If 4.65 moles of propane, C3H8, are b...
Questions

Mathematics, 30.04.2021 17:30


Physics, 30.04.2021 17:30



Computers and Technology, 30.04.2021 17:30

Chemistry, 30.04.2021 17:30

Law, 30.04.2021 17:30




Physics, 30.04.2021 17:30



Mathematics, 30.04.2021 17:30



Arts, 30.04.2021 17:30


Social Studies, 30.04.2021 17:30

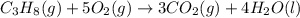
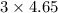 moles of carbon dioxide will form
moles of carbon dioxide will form 

