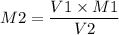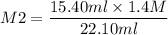If I have 15.40mL of 1.4M HBr(aq) and add 22.10mL KOH what is the molarity of
KOH being used?...

Chemistry, 07.04.2020 21:31 noathequeen
If I have 15.40mL of 1.4M HBr(aq) and add 22.10mL KOH what is the molarity of
KOH being used?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1


Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1
You know the right answer?
Questions

Advanced Placement (AP), 29.04.2021 07:10

Mathematics, 29.04.2021 07:10

Mathematics, 29.04.2021 07:10

Advanced Placement (AP), 29.04.2021 07:10

Mathematics, 29.04.2021 07:10


Mathematics, 29.04.2021 07:10

Mathematics, 29.04.2021 07:10

Health, 29.04.2021 07:10

Mathematics, 29.04.2021 07:10

Mathematics, 29.04.2021 07:10

History, 29.04.2021 07:10

English, 29.04.2021 07:10


English, 29.04.2021 07:10

Spanish, 29.04.2021 07:10

Physics, 29.04.2021 07:10


English, 29.04.2021 07:10