

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
How can you use chemical equations to predict the products of the reaction you can carry out?
Answers: 1



You know the right answer?
When the experiment was carried out, the temperature was 22 oC and atmospheric pressure was 1.007 at...
Questions



English, 20.08.2020 18:01

Computers and Technology, 20.08.2020 18:01










Mathematics, 20.08.2020 18:01






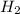 =4.16mol
=4.16mol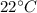 =273+22 k=295k
=273+22 k=295k

