
Chemistry, 07.04.2020 21:50 hunteryolanda82
The Kb of weak base B is 1.0 x 10-6. A solution contains 0.75 M B and 0.25 M HB+Cl- (the salt of B with HCl). What is the pH of the solution after 0.05 mol NaOH is added to 1.0 L of the above solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1



Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
The Kb of weak base B is 1.0 x 10-6. A solution contains 0.75 M B and 0.25 M HB+Cl- (the salt of B w...
Questions


Mathematics, 13.05.2021 20:30


Mathematics, 13.05.2021 20:30


History, 13.05.2021 20:30

Physics, 13.05.2021 20:30



Mathematics, 13.05.2021 20:30

Mathematics, 13.05.2021 20:30


Mathematics, 13.05.2021 20:30


History, 13.05.2021 20:30





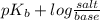
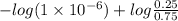
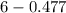
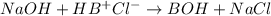
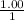 , [Salt] =
, [Salt] = 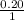
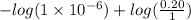 [/tex]
[/tex]

