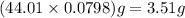Gaseous methane (CH4) reacts with gaseous oxygen (O2) gas to produce gaseous carbon dioxide (CO2) and gaseous water (H2O). What is the theoretical yield of carbon dioxide formed from the reaction of 1.28 g of methane and 10.1 g of oxygen gas? Be sure your answer has the correct number of significant digits in it.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
Gaseous methane (CH4) reacts with gaseous oxygen (O2) gas to produce gaseous carbon dioxide (CO2) an...
Questions

Social Studies, 17.12.2020 18:40

English, 17.12.2020 18:40




Mathematics, 17.12.2020 18:40

Mathematics, 17.12.2020 18:40

Chemistry, 17.12.2020 18:40





Mathematics, 17.12.2020 18:40


Arts, 17.12.2020 18:40





English, 17.12.2020 18:40

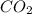 is 3.51 g.
is 3.51 g.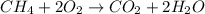
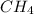 = 16.04 g/mol
= 16.04 g/mol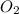 = 32.00 g/mol
= 32.00 g/mol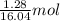 of
of 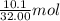 of
of