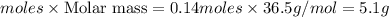Chemistry, 07.04.2020 21:05 laura52677
G Aqueous hydrochloric acid will react with solid sodium hydroxide to produce aqueous sodium chloride and liquid water . Suppose 20. g of hydrochloric acid is mixed with 16.3 g of sodium hydroxide. Calculate the minimum mass of hydrochloric acid that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
G Aqueous hydrochloric acid will react with solid sodium hydroxide to produce aqueous sodium chlorid...
Questions

Engineering, 16.08.2019 18:10

Mathematics, 16.08.2019 18:10



Health, 16.08.2019 18:10

Mathematics, 16.08.2019 18:10

Advanced Placement (AP), 16.08.2019 18:10

Mathematics, 16.08.2019 18:10




Chemistry, 16.08.2019 18:10

Business, 16.08.2019 18:10

Mathematics, 16.08.2019 18:10


English, 16.08.2019 18:10

Physics, 16.08.2019 18:10


Mathematics, 16.08.2019 18:10

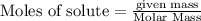
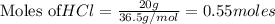
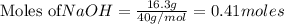
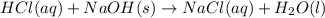
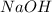 require = 1 mole of
require = 1 mole of 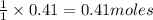 of
of 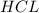 is the excess reagent.
is the excess reagent.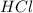 left =
left =