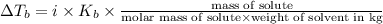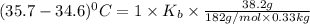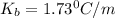Chemistry, 07.04.2020 21:15 esanchez2002fcb
What is the boiling point elevation constant, Kb, of diethyl ether if 38.2 g of the nonelectrolyte benzophenone, C6H5COC6H5, dissolved in 330. g of diethyl ether produces a solution that boils at 35.7°C? Use molar masses with at least as many significant figures as the data given.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1
You know the right answer?
What is the boiling point elevation constant, Kb, of diethyl ether if 38.2 g of the nonelectrolyte b...
Questions

English, 16.12.2020 20:40

Chemistry, 16.12.2020 20:40

Biology, 16.12.2020 20:40

Mathematics, 16.12.2020 20:40

Social Studies, 16.12.2020 20:40

History, 16.12.2020 20:40


History, 16.12.2020 20:40



Computers and Technology, 16.12.2020 20:40

Mathematics, 16.12.2020 20:40

Mathematics, 16.12.2020 20:40

Mathematics, 16.12.2020 20:40



Mathematics, 16.12.2020 20:40


Mathematics, 16.12.2020 20:40

Advanced Placement (AP), 16.12.2020 20:40

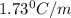
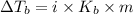
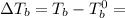 = Elevation in boling point
= Elevation in boling point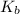 =boiling point constant = ?
=boiling point constant = ?