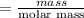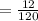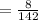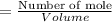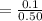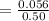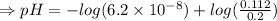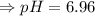Chemistry, 07.04.2020 20:14 raekwonpowell10
6. What is the pH of the buffer that results when 12.0 g of NaH2PO4 and 8.00 g of Na2HPO4 are diluted with water to a volume of 0.50 L? (Ka of H2PO4– = 6.2 x10–8, the molar masses of NaH2PO4 and Na2HPO4 are 120.0 g/mol and 142.0 mol, respectively)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1



Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
6. What is the pH of the buffer that results when 12.0 g of NaH2PO4 and 8.00 g of Na2HPO4 are dilute...
Questions


Computers and Technology, 02.03.2021 17:30




History, 02.03.2021 17:30




Mathematics, 02.03.2021 17:30

Mathematics, 02.03.2021 17:30

English, 02.03.2021 17:30



Mathematics, 02.03.2021 17:30



Mathematics, 02.03.2021 17:30


English, 02.03.2021 17:30

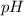 of the buffer solution is 6.96.
of the buffer solution is 6.96.![pH= pK_a+log \frac{[salt]}{[acid]}](/tpl/images/0587/1250/39469.png) .
.