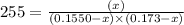For the following reaction, KcKc = 255 at 1000 KK.
CO (g) + Cl2 (g) ⇌ COCl2 (g)CO (g) + Cl2 (g) ⇌ COCl2 (g)
A reaction mixture initially contains a COCO concentration of 0.1550 MM and a Cl2Cl2 concentration of 0.173 MM at 1000 KK.
a) What is the equilibrium concentration of COCO at 1000 KK?
b) What is the equilibrium concentration of Cl2Cl2 at 1000 KK?
c) What is the equilibrium concentration of COCl2COCl2 at 1000 KK?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

Chemistry, 23.06.2019 08:30
What percentage of energy used in the u.s is produced from fossil fuels
Answers: 2
You know the right answer?
For the following reaction, KcKc = 255 at 1000 KK.
CO (g) + Cl2 (g) ⇌ COCl2 (g)CO (g) + Cl2 (g...
CO (g) + Cl2 (g) ⇌ COCl2 (g)CO (g) + Cl2 (g...
Questions

Mathematics, 21.07.2021 21:20

English, 21.07.2021 21:20


History, 21.07.2021 21:20








Computers and Technology, 21.07.2021 21:20


Mathematics, 21.07.2021 21:20

Mathematics, 21.07.2021 21:20

Mathematics, 21.07.2021 21:20

Social Studies, 21.07.2021 21:20

History, 21.07.2021 21:20

Mathematics, 21.07.2021 21:20

Health, 21.07.2021 21:20

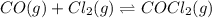

![K_c=\frac{[COCl_2]}{[CO][Cl_2]}](/tpl/images/0587/0731/36d91.png)