

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the following properties was originally used to arrange elements on the periodic table, but is no longer used to organize the modern version. which property fits this description?
Answers: 3

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
A sample of argon gas, Ar(g). is placed in a 3.80 L container at 320 K. The gas pressure is 0.496 at...
Questions

Mathematics, 01.04.2020 06:20


Mathematics, 01.04.2020 06:20


Mathematics, 01.04.2020 06:20





Mathematics, 01.04.2020 06:21

Mathematics, 01.04.2020 06:21

Spanish, 01.04.2020 06:21

Mathematics, 01.04.2020 06:21

Biology, 01.04.2020 06:21


Mathematics, 01.04.2020 06:21


Mathematics, 01.04.2020 06:21

Chemistry, 01.04.2020 06:21

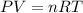 .......(1)
.......(1)


