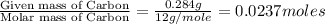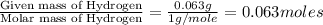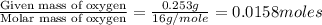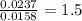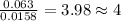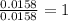Chemistry, 07.04.2020 17:26 Crtive6538
Combustion analysis of 0.600 g of an unknown compound containing carbon, hydrogen, and oxygen produced 1.043 g of CO2 and 0.5670 g of H2O. What is the empirical formula of the compound? Note because you are not able to enter subscripts enter the answer in the form: CxHyOz

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2


Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2
You know the right answer?
Combustion analysis of 0.600 g of an unknown compound containing carbon, hydrogen, and oxygen produc...
Questions



History, 26.10.2020 18:50

Advanced Placement (AP), 26.10.2020 18:50


Mathematics, 26.10.2020 18:50



English, 26.10.2020 18:50


History, 26.10.2020 18:50




Mathematics, 26.10.2020 18:50




English, 26.10.2020 18:50

Mathematics, 26.10.2020 18:50

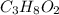
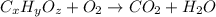
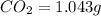
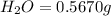
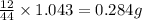 of carbon will be contained.
of carbon will be contained.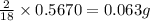 of hydrogen will be contained.
of hydrogen will be contained.