

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 04:00
If you are told to get 100 ml of stock solution to use to prepare smaller size sample for an experiment, which piece of glassware would you use?
Answers: 3
You know the right answer?
2) Give the number of lone pairs around the central atom and the molecular geometry of IF5. A) 0 lon...
Questions





English, 05.02.2021 21:00



History, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00

Chemistry, 05.02.2021 21:00

Chemistry, 05.02.2021 21:00

Mathematics, 05.02.2021 21:00


Mathematics, 05.02.2021 21:00

English, 05.02.2021 21:00





Biology, 05.02.2021 21:00

![\text{Number of electrons}=\frac{1}{2}[V+N-C+A]](/tpl/images/0586/4224/86ecf.png)
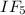 molecule.
molecule.![\text{Number of electrons}=\frac{1}{2}\times [7+5+0-0]=6](/tpl/images/0586/4224/6e043.png)
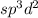 and the electronic geometry of the molecule will be octahedral .
and the electronic geometry of the molecule will be octahedral . 

