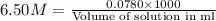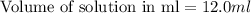Chemistry, 07.04.2020 15:52 boweytom6217
Zinc reacts with hydrochloric acid according to the reaction equation Zn ( s ) + 2 HCl ( aq ) ⟶ ZnCl 2 ( aq ) + H 2 ( g ) How many milliliters of 6.50 M HCl ( aq ) are required to react with 2.55 g Zn ( s ) ?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
Zinc reacts with hydrochloric acid according to the reaction equation Zn ( s ) + 2 HCl ( aq ) ⟶ ZnCl...
Questions






Mathematics, 25.02.2020 03:27







English, 25.02.2020 03:27

Mathematics, 25.02.2020 03:27





Mathematics, 25.02.2020 03:27

Mathematics, 25.02.2020 03:27

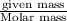
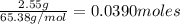
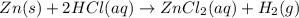
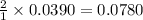 moles of HCl
moles of HCl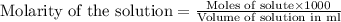 .....(1)
.....(1)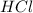 solution = 6.50 M
solution = 6.50 M