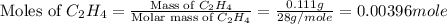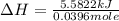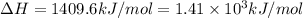Chemistry, 07.04.2020 15:12 kimberlylove387
A bomb calorimeter has a heat capacity of 2.47 kJ/K including the water. When a 0.111 g sample of ethylene (C 2H 4) was burned in this calorimeter, the temperature increased by 2.26 K. Calculate the energy of combustion for one mole of ethylene. a. -50.3 kJ/mol b. -1.41 x 103 kJ/mol c. -0.274 kJ/mol d. -624 kJ/mol e. -5.29 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2
You know the right answer?
A bomb calorimeter has a heat capacity of 2.47 kJ/K including the water. When a 0.111 g sample of et...
Questions




Mathematics, 26.07.2019 07:10



Mathematics, 26.07.2019 07:10

Biology, 26.07.2019 07:10


Mathematics, 26.07.2019 07:10

Mathematics, 26.07.2019 07:10

English, 26.07.2019 07:10

Mathematics, 26.07.2019 07:10

Chemistry, 26.07.2019 07:10

English, 26.07.2019 07:10





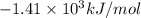
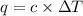
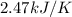
 = Change in temperature = 2.26 K
= Change in temperature = 2.26 K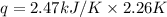
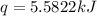
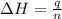
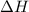 = energy of combustion for one mole of ethylene = ?
= energy of combustion for one mole of ethylene = ?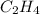 = 0.111 g
= 0.111 g