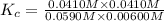Chemistry, 07.04.2020 03:15 sethlynn2003
Calculate the equilibrium constant for the reaction using the balanced chemical equation and the concentrations of the substances at equilibrium.
Use the
appropriate significant figures in reporting the answers.
CO(g) + H2O(g) ⇌ CO2(g) + H2(g) [CO] = 0.0590 M; [H2O] = 0.00600 M;
[CO2] = 0.0410 M; [H2] = 0.0410 M
K =-

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3


Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1
You know the right answer?
Calculate the equilibrium constant for the reaction using the balanced chemical equation and the con...
Questions


Mathematics, 17.10.2020 14:01

History, 17.10.2020 14:01



Advanced Placement (AP), 17.10.2020 14:01



World Languages, 17.10.2020 14:01

Health, 17.10.2020 14:01





Chemistry, 17.10.2020 14:01

History, 17.10.2020 14:01

Biology, 17.10.2020 14:01

Mathematics, 17.10.2020 14:01

Advanced Placement (AP), 17.10.2020 14:01

Arts, 17.10.2020 14:01

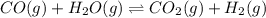
![[CO]=0.0590 M,[H_2O]=0.00600 M](/tpl/images/0585/8718/63397.png)
![[CO_2]=0.0410 M,[H_2]=0.0410 M](/tpl/images/0585/8718/efcda.png)
![K_c=\frac{[CO_2][H_2]}{[CO][H_2O]}](/tpl/images/0585/8718/fbbde.png)