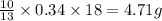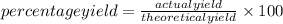Chemistry, 07.04.2020 01:38 vanessa791
Gaseous butane CH3(CH2)2CH3 reacts with gaseous oxygen gas O2 to produce gaseous carbon dioxide CO2 and gaseous water H2O. If 1.31g of water is produced from the reaction of 4.65g of butane and 10.8g of oxygen gas, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
There is an area in idaho named craters of the moon where most of the ground is covered with basalt, adark gray, igneous rock with no visibl crystals. what can you infer about the geographical history of the area?
Answers: 1

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

You know the right answer?
Gaseous butane CH3(CH2)2CH3 reacts with gaseous oxygen gas O2 to produce gaseous carbon dioxide CO2...
Questions


History, 15.01.2021 02:30


Mathematics, 15.01.2021 02:30

Mathematics, 15.01.2021 02:30

History, 15.01.2021 02:30

Mathematics, 15.01.2021 02:30

Mathematics, 15.01.2021 02:30

History, 15.01.2021 02:30

History, 15.01.2021 02:30

History, 15.01.2021 02:30


French, 15.01.2021 02:30

History, 15.01.2021 02:30

Chemistry, 15.01.2021 02:30


Mathematics, 15.01.2021 02:30

Mathematics, 15.01.2021 02:30


Mathematics, 15.01.2021 02:30

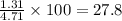
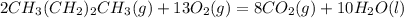
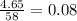
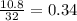
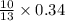 mole of water.
mole of water.