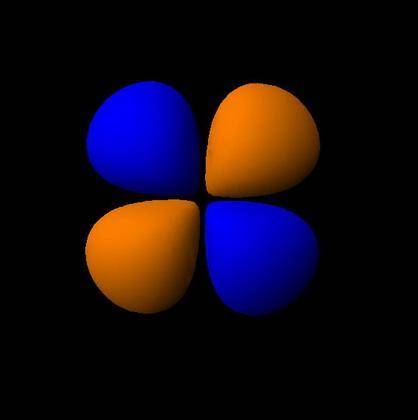Chemistry, 07.04.2020 00:47 glowbaby123
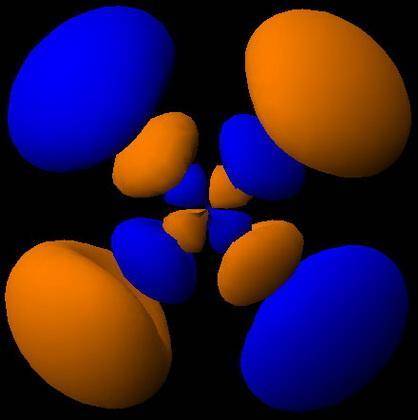
How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 shell?
Determine which statement is true or false.
The orbital in the n = 5 shell is bigger than the orbital in the n = 3 shell.
The value of l would increase by 2 for the n = 5 shell.
The value of l for both orbitals would be the same.
The orientation of the n = 5 orbital would be rotated 45∘ along the xy plane.
The mâ„“ value for both orbitals would be the same.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 23.06.2019 02:00
What are fossils of organisms that existed over a wide area but only for a limited time period called?
Answers: 2
You know the right answer?
How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 shell?
Questions





Computers and Technology, 01.12.2021 22:20

Mathematics, 01.12.2021 22:20

Mathematics, 01.12.2021 22:20


Business, 01.12.2021 22:20



History, 01.12.2021 22:20

Mathematics, 01.12.2021 22:20


Biology, 01.12.2021 22:20


History, 01.12.2021 22:20


Computers and Technology, 01.12.2021 22:20