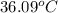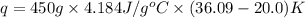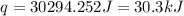150.0 mL of 2.00 M Pb(NO3)2 solution is mixed with 300.0 mL of 2.00 M NaI solution in a coffee cup calorimeter of negligible heat capacity.
The initial temperature of the two solutions are both at 20.00 oC, the final temperature of the mixed solution is 36.09 oC.
c of H 2O = 4.184 J/g oC ; d of solution is 1.00g/mL. Refer to the following:
Pb(NO3)2(aq) + 2NaI (aq) --> PbI2(s) + 2NaNO3(aq)
What is q of the water? Please watch units. Answers have units of kJ. You have to convert J to kJ.
-1790 kJ/mol
42.9 kJ/mol
- 451 kJ/mol
30.3 kJ/mol
20.9 kJ/mol
91.7 kJ/mol
85.4 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3


Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
150.0 mL of 2.00 M Pb(NO3)2 solution is mixed with 300.0 mL of 2.00 M NaI solution in a coffee cup c...
Questions

Mathematics, 13.12.2020 08:40


Mathematics, 13.12.2020 08:40



History, 13.12.2020 08:40



Physics, 13.12.2020 08:40



Mathematics, 13.12.2020 08:40

Biology, 13.12.2020 08:40

Mathematics, 13.12.2020 08:40

Mathematics, 13.12.2020 08:40

Mathematics, 13.12.2020 08:40

Biology, 13.12.2020 08:40



Mathematics, 13.12.2020 08:40

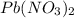 and NaI.
and NaI.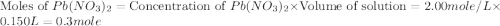
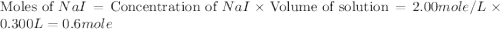
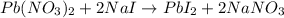
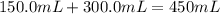
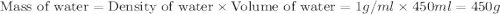
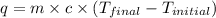
 = specific heat of water =
= specific heat of water = 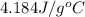
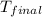 = final temperature of water =
= final temperature of water = 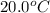
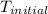 = initial temperature of metal =
= initial temperature of metal =