
Chemistry, 06.04.2020 22:19 birdwithpurpleboots
Consider a mixture of air and gasoline vapor in a cylinder with a piston. The original volume is 0.09 L. If the combustion of this mixture releases 900. Jof energy, to what volume will the gases expand against a constant pressure of 670. torr if all the energy of combustion is converted into work to push back the piston? (1 atm = 760 torr, 1 L atm = 101.325 J) a. 10.00 L b. 10.17 L c. 1.47 L d. 1.34L

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
Consider a mixture of air and gasoline vapor in a cylinder with a piston. The original volume is 0.0...
Questions

Mathematics, 04.06.2020 13:17


Mathematics, 04.06.2020 13:17


English, 04.06.2020 13:17

Mathematics, 04.06.2020 13:17


History, 04.06.2020 13:17









Mathematics, 04.06.2020 13:18


History, 04.06.2020 13:18

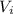 = 0.09 L, P = 670 torr
= 0.09 L, P = 670 torr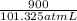
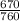 atm
atm
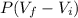
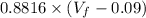
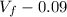 = 10.08
= 10.08
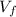 = 10.17 L
= 10.17 L

