
Chemistry, 06.04.2020 19:44 pollywallythecat
A 0.22 −mol sample of a weak acid with an unknown pKa was combined with 12.0 mL of 3.20 M KOH and the resulting solution was diluted to 1.500 L. The measured pH of the solution was 3.90. What is the pka of the weak acid?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 11:20
Sandy is building a small toy car. he wants to use a balloon to power the toy car. he fills a balloon with air and then attaches a straw to the balloon. he tapes the balloon-straw combination to the car and then releases the end of the balloon. the toy moves forward as the air from the balloon comes out the back of the straw. what can sandy do to make the toy car move faster? a) use less air in the balloon. b) blow up the balloon more. c) use a longer straw. d) use larger tires.
Answers: 2
You know the right answer?
A 0.22 −mol sample of a weak acid with an unknown pKa was combined with 12.0 mL of 3.20 M KOH and...
Questions

Mathematics, 19.10.2020 01:01


History, 19.10.2020 01:01



Arts, 19.10.2020 01:01

Biology, 19.10.2020 01:01

Biology, 19.10.2020 01:01

Mathematics, 19.10.2020 01:01



Mathematics, 19.10.2020 01:01

Mathematics, 19.10.2020 01:01




Mathematics, 19.10.2020 01:01

Spanish, 19.10.2020 01:01

English, 19.10.2020 01:01

English, 19.10.2020 01:01

![pH=pKa+log(\frac{[A^{-} ]}{[HA]} )](/tpl/images/0584/7613/35476.png) --------------------(i)
--------------------(i)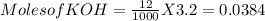
![pKa = pH - log(\frac{[A^{-} ]}{[HA]} )\\\\ =3.9 - log(\frac{0.0384}{0.2116} )\\\\= 3.9 - log (0.18)\\\\= 3.9+0.74\\\\=4.64](/tpl/images/0584/7613/60c1a.png)


