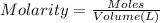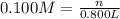Chemistry, 06.04.2020 18:54 devinmoore4664
A chemist must prepare of 800.0 ml potassium hydroxide solution with a pH of 13.00 at 25°.
She will do this in three steps:
Fill a 800 ml volumetric flask about halfway with distilled water.
Weigh out a small amount of solid potassium hydroxide and add it to the flask.
Fill the flask to the mark with distilled water.
Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1


Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
A chemist must prepare of 800.0 ml potassium hydroxide solution with a pH of 13.00 at 25°.
Questions

Social Studies, 07.07.2019 21:30

Mathematics, 07.07.2019 21:30



Mathematics, 07.07.2019 21:30

Social Studies, 07.07.2019 21:30


Physics, 07.07.2019 21:30


Social Studies, 07.07.2019 21:30

Mathematics, 07.07.2019 21:30





Biology, 07.07.2019 21:30

Chemistry, 07.07.2019 21:30

Mathematics, 07.07.2019 21:30

Business, 07.07.2019 21:30

![pOH=-\log[OH^-]](/tpl/images/0584/6258/fe336.png)
![1.00=-\log[OH^-]](/tpl/images/0584/6258/6b8f7.png)
![[OH^-]=10^{-1.00} M=0.100 M](/tpl/images/0584/6258/a9d8c.png)
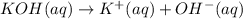
![[KOH]=[OH^-]=[K^+]=0.100 M](/tpl/images/0584/6258/fca0f.png)