
Chemistry, 06.04.2020 11:30 justice808
4. Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide. Assume all gases are at the same conditions of temperature and pressure.
2N2(g) + 3O2(g) 2N2O3(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

You know the right answer?
4. Calculate the number of liters of oxygen gas needed to produce 15.0 liters of dinitrogen trioxide...
Questions


Biology, 24.10.2020 05:00



English, 24.10.2020 05:10

Mathematics, 24.10.2020 05:10

Mathematics, 24.10.2020 05:10




Mathematics, 24.10.2020 05:10


Mathematics, 24.10.2020 05:10

Mathematics, 24.10.2020 05:10

Mathematics, 24.10.2020 05:10

Mathematics, 24.10.2020 05:10



Mathematics, 24.10.2020 05:10

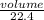
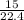 = 0.67moles
= 0.67moles 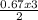 = 1.01 moles of O₂
= 1.01 moles of O₂

