

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1
You know the right answer?
The enthalpy change for converting 10.0 g of ice at -25.0 °c to water at 90.0 °c is kj. the specifi...
Questions

Mathematics, 23.11.2019 14:31


Biology, 23.11.2019 14:31

English, 23.11.2019 14:31

Mathematics, 23.11.2019 14:31

Chemistry, 23.11.2019 14:31




Physics, 23.11.2019 14:31

Social Studies, 23.11.2019 14:31

History, 23.11.2019 14:31

Mathematics, 23.11.2019 14:31

Geography, 23.11.2019 14:31

English, 23.11.2019 14:31

Arts, 23.11.2019 14:31

Biology, 23.11.2019 14:31



French, 23.11.2019 14:31

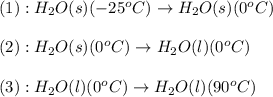
![\Delta H=[m\times c_{p,s}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[m\times c_{p,l}\times (T_{final}-T_{initial})]](/tpl/images/0474/9125/5cd06.png)
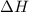 = enthalpy change = ?
= enthalpy change = ?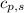 = specific heat of solid water =
= specific heat of solid water = 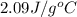
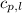 = specific heat of liquid water =
= specific heat of liquid water = 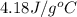
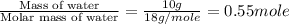
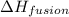 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole![\Delta H=[10g\times 4.18J/gK\times (0-(-25))^oC]+0.55mole\times 6010J/mole+[10g\times 2.09J/gK\times (90-0)^oC]](/tpl/images/0474/9125/4de79.png)
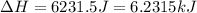 (1 KJ = 1000 J)
(1 KJ = 1000 J)

