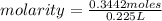Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2


Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
What is the molarity of 225 mL of a KNO3 solution containing 34.8 g KNO3? (molar mass = 101.1 g/mol)...
Questions

Biology, 07.05.2021 16:00


English, 07.05.2021 16:00

Mathematics, 07.05.2021 16:00

Mathematics, 07.05.2021 16:00


Mathematics, 07.05.2021 16:00



English, 07.05.2021 16:00



Mathematics, 07.05.2021 16:00

History, 07.05.2021 16:00

Mathematics, 07.05.2021 16:00

Mathematics, 07.05.2021 16:00


Mathematics, 07.05.2021 16:00

History, 07.05.2021 16:00

Social Studies, 07.05.2021 16:00

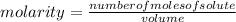
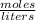 .
.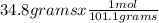 = 0.3442 moles, being 101.1
= 0.3442 moles, being 101.1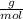 , the molar mass of the compound, that is, the amount of mass that a substance contains in one mole.volume= 225 mL= 0.225 L (being 1000 mL= 1 L)
, the molar mass of the compound, that is, the amount of mass that a substance contains in one mole.volume= 225 mL= 0.225 L (being 1000 mL= 1 L)