
2 Points
Which of the following is the correct equation for the reaction below?
One molecule of methane gas reacts with two molecules of oxygen gas to
form one molecule of carbon dioxide gas and two molecules of water vapor.
O
A. CH (g) + O2 (g) - CO (g) + H20 (g)
O B. CH (9) + 20 (g) → CO(g) + 2H0 (9)
O C. CH4 (g) + 2O2 (g) – có (g) + 2H20 (g)
OD. CHA (9) + 202 (9) ► 02 (9) + H20 (9)
Need ASAP

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3


Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
2 Points
Which of the following is the correct equation for the reaction below?
One mole...
Which of the following is the correct equation for the reaction below?
One mole...
Questions

Mathematics, 11.10.2019 22:30

Spanish, 11.10.2019 22:30

History, 11.10.2019 22:30





Mathematics, 11.10.2019 22:30

Biology, 11.10.2019 22:30

Advanced Placement (AP), 11.10.2019 22:30


English, 11.10.2019 22:30

Social Studies, 11.10.2019 22:30




Mathematics, 11.10.2019 22:30



Mathematics, 11.10.2019 22:30

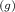 + 2O₂
+ 2O₂

