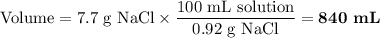Chemistry, 05.04.2020 20:50 blancasimon239
A saline solution used in intravenous drips for patients who cannot take oral fluids contains 0.92% NaCl in water. What volume of the saline solution must be administered to the patient in order to deliver 7.7g of NaCl?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2
You know the right answer?
A saline solution used in intravenous drips for patients who cannot take oral fluids contains 0.92%...
Questions




History, 19.01.2021 19:50

Mathematics, 19.01.2021 19:50




Mathematics, 19.01.2021 19:50


Mathematics, 19.01.2021 19:50

English, 19.01.2021 19:50

Chemistry, 19.01.2021 19:50

Mathematics, 19.01.2021 19:50

Computers and Technology, 19.01.2021 19:50