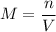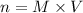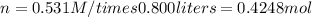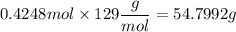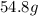Chemistry, 05.04.2020 17:39 laceysmith2i023
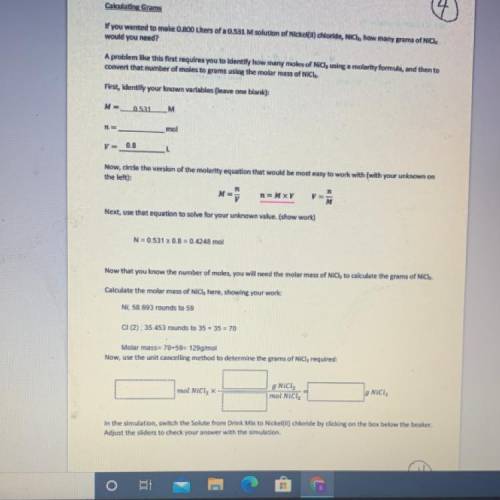
If you wanted to make 0.800 Liters of a 0.531 M solution of Nickel(II) chloride, NiCl2, how many grams of NiCIZ
would you need? Use the unit canceling method to determine the grams of NiCl2
Molar mass= 129g/mol. Moles = 0.4248
.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2


Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2
You know the right answer?
If you wanted to make 0.800 Liters of a 0.531 M solution of Nickel(II) chloride, NiCl2, how many gra...
Questions

English, 10.09.2021 03:10

Mathematics, 10.09.2021 03:10


English, 10.09.2021 03:10

English, 10.09.2021 03:10

Mathematics, 10.09.2021 03:10

English, 10.09.2021 03:10




Mathematics, 10.09.2021 03:10

Mathematics, 10.09.2021 03:10

Mathematics, 10.09.2021 03:10



Mathematics, 10.09.2021 03:10



Mathematics, 10.09.2021 03:10

Mathematics, 10.09.2021 03:10