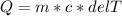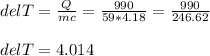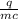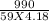JC and D
If 990.J of heat is absorbed by a 59 g sample of water at 21.0 °C, what
will the final temperature of this sample be after absorbing this energy
The specific heat of water is 4.18 J/9g °C). *
25 degrees Celsius
O
35 degrees Celsius
o
2.5 degrees Celsius

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

You know the right answer?
JC and D
If 990.J of heat is absorbed by a 59 g sample of water at 21.0 °C, what
will th...
If 990.J of heat is absorbed by a 59 g sample of water at 21.0 °C, what
will th...
Questions


Mathematics, 22.04.2020 00:49




Mathematics, 22.04.2020 00:49






Mathematics, 22.04.2020 00:50

Mathematics, 22.04.2020 00:50

Mathematics, 22.04.2020 00:50

Mathematics, 22.04.2020 00:50

Health, 22.04.2020 00:50

History, 22.04.2020 00:50

Mathematics, 22.04.2020 00:50