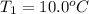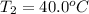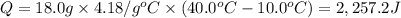Chemistry, 05.04.2020 03:16 meganwintergirl
Calculate the energy needed to raise the temperature of 18.0g of water from 10.0C to 40.0C. The specific heat of water is 4.18 J/gC. *

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1
You know the right answer?
Calculate the energy needed to raise the temperature of 18.0g of water from 10.0C to 40.0C. The spec...
Questions








Computers and Technology, 31.03.2020 00:23

Computers and Technology, 31.03.2020 00:23



Mathematics, 31.03.2020 00:23








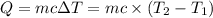
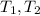 : Initial and final temperature of the substance
: Initial and final temperature of the substance