
Chemistry, 04.04.2020 21:52 Shybaby5019
Sodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution contains 521 g of NaOH(s) per liter of solution. Calculate the molarity of this saturated NaOH(aq) solution.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3

Chemistry, 23.06.2019 09:30
How many moles of na2s2o3 are needed to react with 0.12mol of cl2? show work.
Answers: 1

You know the right answer?
Sodium hydroxide is extremely soluble in water. At a certain temperature, a saturated solution conta...
Questions










English, 27.02.2020 01:51

Mathematics, 27.02.2020 01:51



Mathematics, 27.02.2020 01:51

Mathematics, 27.02.2020 01:51


Computers and Technology, 27.02.2020 01:51



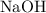 solution is approximately
solution is approximately 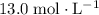 .
.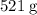 of
of 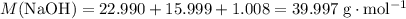 .
.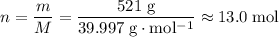 .
.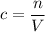 to find the molar concentration
to find the molar concentration  of this solution. In this equation,
of this solution. In this equation, 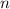 is the number of moles of the solute, and
is the number of moles of the solute, and 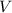 is the volume of the solution.
is the volume of the solution.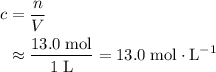 .
. 


