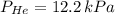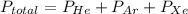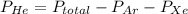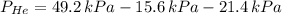Chemistry, 04.04.2020 21:30 maisonsuperman5321
A mixture of helium, argon, and xenon gases are present in a container. What is the partial pressure of helium if the total pressure is 49.2 kPa and the partial pressure of Ar is 15.6 kPa and 21,4 kPa for Xe?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 02:00
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2
You know the right answer?
A mixture of helium, argon, and xenon gases are present in a container. What is the partial pressure...
Questions


Mathematics, 23.03.2021 18:50

Mathematics, 23.03.2021 18:50

Mathematics, 23.03.2021 18:50




Mathematics, 23.03.2021 18:50


Mathematics, 23.03.2021 18:50




English, 23.03.2021 18:50

Mathematics, 23.03.2021 18:50


Mathematics, 23.03.2021 18:50

Mathematics, 23.03.2021 18:50

Arts, 23.03.2021 18:50

Mathematics, 23.03.2021 18:50