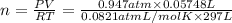Chemistry, 04.04.2020 20:50 heavendl13
A chemist reacts magnesium with hydrochloric acid and collects 57.48 mL of the hydrogen gas that is produced by water displacement. If the lab temperature is 24 C and the atmospheric pressure is 742.1 mm Hg, how many grams of hydrogen are produced? Water vapor pressure is 22.4 mm Hg at 24 C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1


Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
A chemist reacts magnesium with hydrochloric acid and collects 57.48 mL of the hydrogen gas that is...
Questions

English, 13.11.2020 23:10

Mathematics, 13.11.2020 23:10

History, 13.11.2020 23:10

Mathematics, 13.11.2020 23:10

Mathematics, 13.11.2020 23:10

Chemistry, 13.11.2020 23:10



English, 13.11.2020 23:10

Mathematics, 13.11.2020 23:10



History, 13.11.2020 23:10

Chemistry, 13.11.2020 23:10

Mathematics, 13.11.2020 23:10


History, 13.11.2020 23:10


English, 13.11.2020 23:10

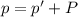
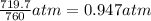
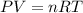 ( Ideal gas equation)
( Ideal gas equation)