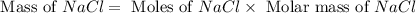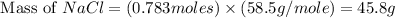2Na+Cl 2 →2NaCl2, start text, N, a, end text, plus, start text, C, l, end text, start subscript, 2, end subscript, right arrow, 2, start text, N, a, C, l, end text How many grams of \text{NaCl}NaClstart text, N, a, C, l, end text will be produced from 18.0 \text{ g}18.0 g18, point, 0, start text, space, g, end text of \text{Na}Nastart text, N, a, end text and 23.0 \text{ g}23.0 g23, point, 0, start text, space, g, end text of \text{Cl}_2Cl 2 start text, C, l, end text, start subscript, 2, end subscript?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3
You know the right answer?
2Na+Cl 2 →2NaCl2, start text, N, a, end text, plus, start text, C, l, end text, start subscript, 2,...
Questions


Chemistry, 30.01.2020 01:54

Computers and Technology, 30.01.2020 01:54

Mathematics, 30.01.2020 01:55

Biology, 30.01.2020 01:55


Mathematics, 30.01.2020 01:55

Mathematics, 30.01.2020 01:55

Mathematics, 30.01.2020 01:55

Mathematics, 30.01.2020 01:55



Mathematics, 30.01.2020 01:55

Biology, 30.01.2020 01:55



Mathematics, 30.01.2020 01:55

Mathematics, 30.01.2020 01:55

English, 30.01.2020 01:55

Mathematics, 30.01.2020 01:55

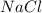 produced is, 45.8 grams.
produced is, 45.8 grams.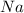 = 18.0 g
= 18.0 g = 23.0 g
= 23.0 g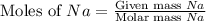
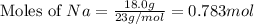
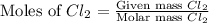


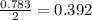 moles of
moles of