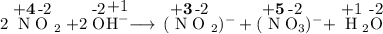Which type of reaction occurs in the following equation?
2NO2(g) + 2OH"(aq) —— NO2(aq) + NO, (...

Chemistry, 04.04.2020 18:10 kimjooin02
Which type of reaction occurs in the following equation?
2NO2(g) + 2OH"(aq) —— NO2(aq) + NO, (aq) +H2O(1)
a combination redox reaction
a displacement redox reaction
a synthetic redox reaction
a disproportionation redox reaction

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
Questions

Social Studies, 31.01.2022 18:20



Mathematics, 31.01.2022 18:20

Mathematics, 31.01.2022 18:20


Mathematics, 31.01.2022 18:20



Mathematics, 31.01.2022 18:30

Mathematics, 31.01.2022 18:30

SAT, 31.01.2022 18:30


Social Studies, 31.01.2022 18:30

Mathematics, 31.01.2022 18:30

Social Studies, 31.01.2022 18:30

English, 31.01.2022 18:30

Mathematics, 31.01.2022 18:30

Mathematics, 31.01.2022 18:30