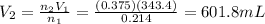Chemistry, 04.04.2020 14:22 gracethegreat1
If 0.214 mol of argon gas occupies a volume of 343.4 mL at a particular temperature and pressure, what volume would 0.375 mol of argon gas occupy under the same conditions?
Answer in - ATM

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1


Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
If 0.214 mol of argon gas occupies a volume of 343.4 mL at a particular temperature and pressure, wh...
Questions



Chemistry, 21.03.2021 19:40

Mathematics, 21.03.2021 19:40

Social Studies, 21.03.2021 19:40

Physics, 21.03.2021 19:40

Social Studies, 21.03.2021 19:40

English, 21.03.2021 19:40

Mathematics, 21.03.2021 19:40

Physics, 21.03.2021 19:40


Computers and Technology, 21.03.2021 19:40

Mathematics, 21.03.2021 19:40

Mathematics, 21.03.2021 19:40

History, 21.03.2021 19:40

Mathematics, 21.03.2021 19:40

English, 21.03.2021 19:40

Mathematics, 21.03.2021 19:40


English, 21.03.2021 19:40

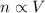
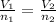
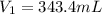 is the initial volume of the gas
is the initial volume of the gas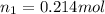 is the initial number of moles
is the initial number of moles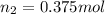 is the final number of moles
is the final number of moles