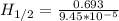Chemistry, 04.04.2020 14:06 munekalove69ounxwv
The isomerization of methylisonitrile to acetonitrile (CH3NC(g) CH3CN) is first order in CH3NC. The rate constant for the reaction is 9.45 x 10-5 s-1 at 478 K. What is the half-life of the reaction when the initial concentration of CH3NC is 0.0300 M?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
The isomerization of methylisonitrile to acetonitrile (CH3NC(g) CH3CN) is first order in CH3NC. The...
Questions


History, 22.10.2020 23:01

Mathematics, 22.10.2020 23:01


English, 22.10.2020 23:01

Mathematics, 22.10.2020 23:01




Mathematics, 22.10.2020 23:01


History, 22.10.2020 23:01

Biology, 22.10.2020 23:01

English, 22.10.2020 23:01

Chemistry, 22.10.2020 23:01

Health, 22.10.2020 23:01

Biology, 22.10.2020 23:01



Mathematics, 22.10.2020 23:01

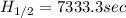
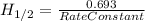
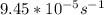 for the rate constant
for the rate constant