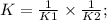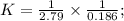Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

You know the right answer?
Two reactions and their equilibrium constants are given. A + 2 B − ⇀ ↽ − 2 C K 1 = 2.79 2 C − ⇀ ↽ −...
Questions







History, 23.09.2019 11:10




Arts, 23.09.2019 11:10



Mathematics, 23.09.2019 11:10

Biology, 23.09.2019 11:10

Biology, 23.09.2019 11:10

Mathematics, 23.09.2019 11:10



Health, 23.09.2019 11:10