
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming of natural gas, in a two-step process. In the first step, nitrogen and hydrogen react to form ammonia: (g)(g)(g) In the second step, ammonia and oxygen react to form nitric acid and water: (g)(g)(g)(g) Write the net chemical equation for the production of nitric acid from nitrogen, hydrogen and oxygen. Be sure your equation is balanced.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
You know the right answer?
Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, plus hydrogen prep...
Questions


Mathematics, 20.06.2020 19:57


History, 20.06.2020 19:57


Mathematics, 20.06.2020 19:57




Mathematics, 20.06.2020 19:57

Health, 20.06.2020 19:57

Computers and Technology, 20.06.2020 19:57

Arts, 20.06.2020 19:57

Mathematics, 20.06.2020 19:57

Mathematics, 20.06.2020 19:57

Mathematics, 20.06.2020 19:57


Computers and Technology, 20.06.2020 19:57


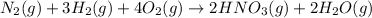
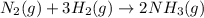 (1)
(1)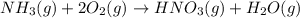
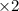
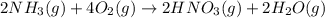 (2)
(2)

