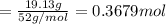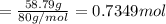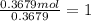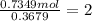Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
A 19.13 gram sample of chromium is heated in the presence of excess bromine. A metal bromide is form...
Questions


History, 15.05.2021 07:10


History, 15.05.2021 07:10




Mathematics, 15.05.2021 07:10

Mathematics, 15.05.2021 07:10


Business, 15.05.2021 07:10



Computers and Technology, 15.05.2021 07:10


Chemistry, 15.05.2021 07:10

World Languages, 15.05.2021 07:10

Mathematics, 15.05.2021 07:10

History, 15.05.2021 07:10

History, 15.05.2021 07:10

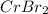 .
.