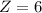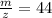Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3
You know the right answer?
Which of the following compounds would be consistent with a compound showing an M with an m/z ratio...
Questions




Mathematics, 02.10.2019 04:00



Biology, 02.10.2019 04:00

Computers and Technology, 02.10.2019 04:00

Mathematics, 02.10.2019 04:00

Mathematics, 02.10.2019 04:00




Business, 02.10.2019 04:00

English, 02.10.2019 04:00


English, 02.10.2019 04:00


Mathematics, 02.10.2019 04:00

English, 02.10.2019 04:00

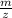 ratio in mass spectrometer,
ratio in mass spectrometer,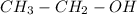
 and atomic number of carbon
and atomic number of carbon