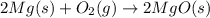Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 3.97 g of magnesium ribbon burns with 8.05 g of oxygen, a bright, white light and a white, powdery product are formed. Enter the balanced chemical equation for this reaction. Be sure to include all physical states.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1


Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. When 3...
Questions

Mathematics, 18.03.2021 22:40


History, 18.03.2021 22:40


Social Studies, 18.03.2021 22:40

History, 18.03.2021 22:40


Mathematics, 18.03.2021 22:40

Mathematics, 18.03.2021 22:40

Physics, 18.03.2021 22:40

Mathematics, 18.03.2021 22:40







Mathematics, 18.03.2021 22:40


Law, 18.03.2021 22:40