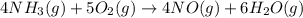Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Strong conductivity of plasma allows it to act and react as and
Answers: 2

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2


Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Gaseous ammonia (NH3) reacts with gaseous oxygen to form gaseous nitrogen monoxide and gaseous water...
Questions

Biology, 10.06.2020 23:57


English, 10.06.2020 23:57

Social Studies, 10.06.2020 23:57


Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57



English, 10.06.2020 23:57

English, 10.06.2020 23:57



Biology, 10.06.2020 23:57


Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57

Mathematics, 10.06.2020 23:57