
Diluting sulfuric acid with water is highly exothermic: (a) Use Appendix B to find for diluting 1.00 mol of H2SO4(l) (d = 1.83 g/mL) to 1 L of 1.00 M H2SO4(aq) (d = 1.060 g/mL). (b) Suppose you carry out the dilution in a calorimeter. The initial T is 25.0°C, and the specific heat capacity of the final solution is 3.50 J/g·K. What is the final T? (c) Use the ideas of density and heat capacity to explain why you should add acid to water rather than water to acid.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 23:00
The data below were determined for the reaction shown below. s2o82– + 3i – (aq) → 2so42– + i3– expt. # [s2o82–] (m) [i –] (m) initial rate 1 0.038 0.060 1.4 × 10 – 5 m/s 2 0.076 0.060 2.8 × 10 – 5 m/s 3 0.076 0.030 1.4 × 10 – 5 m/s the rate law for this reaction must be:
Answers: 1

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1
You know the right answer?
Diluting sulfuric acid with water is highly exothermic: (a) Use Appendix B to find for diluting 1.00...
Questions

Mathematics, 25.11.2020 17:50









Mathematics, 25.11.2020 17:50

History, 25.11.2020 17:50

History, 25.11.2020 17:50

Mathematics, 25.11.2020 17:50

Mathematics, 25.11.2020 17:50






Computers and Technology, 25.11.2020 17:50

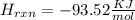
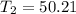 ° c
° c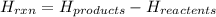
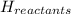 = - 813.9
= - 813.9 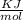
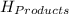 = - 907.51
= - 907.51 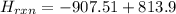
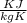
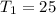 ° c
° c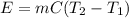
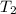 - 298 )
- 298 )

