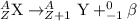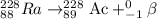Because of the radioactive decay of uranium and thorium in rocks and soil, radium-228, a decay product of Thorium-232, can be found in drinking water. This isotope has a half-life of 5.75 years and an atomic number of 88. If Ra-228 undergoes beta decay, what would the atomic number of the new element be? What would the mass number of this isotope be? Explain your reasoning (e. g. Explain what happens during beta decay).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1
You know the right answer?
Because of the radioactive decay of uranium and thorium in rocks and soil, radium-228, a decay produ...
Questions

English, 06.07.2021 02:50



Social Studies, 06.07.2021 02:50


English, 06.07.2021 02:50

Mathematics, 06.07.2021 02:50


Mathematics, 06.07.2021 02:50



History, 06.07.2021 02:50


Mathematics, 06.07.2021 02:50



Social Studies, 06.07.2021 02:50


Mathematics, 06.07.2021 03:00