
Chemistry, 04.04.2020 11:05 ellaemtagedeane
When iron(III) oxide reacts with aluminum, aluminum oxide and iron are produced. The balanced equation for this reaction is:
Fe2O3 (s) + 2Al (s) > Al2O3 (s) + 2Fe (s)
If 6 moles of aluminum react:
i. The reaction consumes moles of iron(III) oxide.
ii. The reaction produces moles of aluminum oxide and moles of iron.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
When iron(III) oxide reacts with aluminum, aluminum oxide and iron are produced. The balanced equati...
Questions

Mathematics, 06.05.2021 22:40


Mathematics, 06.05.2021 22:40





Mathematics, 06.05.2021 22:40

Mathematics, 06.05.2021 22:40


Mathematics, 06.05.2021 22:40


Mathematics, 06.05.2021 22:40

History, 06.05.2021 22:40

Mathematics, 06.05.2021 22:40

History, 06.05.2021 22:40

Chemistry, 06.05.2021 22:40



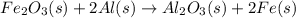
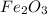
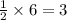 moles of
moles of 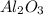
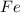
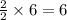 moles of
moles of 

