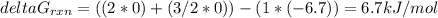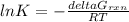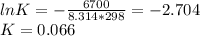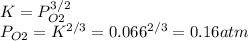Chemistry, 04.04.2020 09:43 emmarieasimon
Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M 3 O 4 ( s ) − ⇀ ↽ − 3 M ( s ) + 2 O 2 ( g ) What is the standard change in Gibbs energy for the reaction, as written, in the forward direction? Δ G ∘ rxn = kJ/mol What is the equilibrium constant of this reaction, as written, in the forward direction at 298 K? K = What is the equilibrium pressure of O2(g) over M(s) at 298 K? P O 2 = atm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2


Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
Consider the decomposition of a metal oxide to its elements, where M represents a generic metal. M 3...
Questions




Mathematics, 15.10.2019 23:50

Mathematics, 15.10.2019 23:50


Mathematics, 15.10.2019 23:50

Advanced Placement (AP), 15.10.2019 23:50


Mathematics, 15.10.2019 23:50

Mathematics, 15.10.2019 23:50

Social Studies, 15.10.2019 23:50



History, 15.10.2019 23:50

Mathematics, 15.10.2019 23:50




Physics, 15.10.2019 23:50