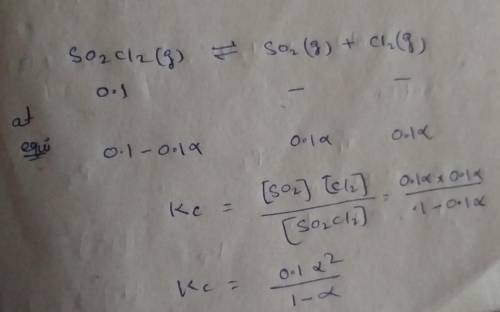Chemistry, 04.04.2020 09:40 kolbehoneyman
Consider the reaction below. Initially the concentration of SO2Cl2 is 0.1000 M. Solve for the equilibrium concentration of SO2Cl2((g). SO2Cl2(g) ←⎯⎯→ SO2(g) + Cl2(g) Kc = 2.99 x 10-7 at 227 °C

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1


Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
You know the right answer?
Consider the reaction below. Initially the concentration of SO2Cl2 is 0.1000 M. Solve for the equili...
Questions

Mathematics, 16.08.2019 20:10

Mathematics, 16.08.2019 20:10

Mathematics, 16.08.2019 20:10




English, 16.08.2019 20:10

Mathematics, 16.08.2019 20:10


History, 16.08.2019 20:10



History, 16.08.2019 20:10

Chemistry, 16.08.2019 20:10

History, 16.08.2019 20:10


Mathematics, 16.08.2019 20:20

Mathematics, 16.08.2019 20:20


![[SO_2Cl_2] = 0.09983 M](/tpl/images/0582/0550/0c89a.png)
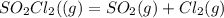
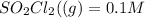
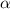
![[SO_2Cl_2] = 0.1-0.1\alpha](/tpl/images/0582/0550/ed240.png)
![[SO_2] = 0.1\alpha](/tpl/images/0582/0550/97c2e.png)
![[Cl_2] = 0.1\alpha](/tpl/images/0582/0550/13ee9.png)
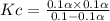
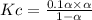

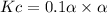
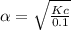
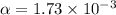
![[SO_2Cl_2] = 0.1-0.1\alpha = 0.1-0.1\times 0.00173](/tpl/images/0582/0550/6431a.png)